Our laboratory addresses the role of innate immune mechanisms in autoimmunity. We have identified an important role for cytosolic DNA sensor pathways in the generation of arthritic inflammation and its subsequent impact on bone. Innate immune pattern recognition receptors sense nucleic acid from microbial organisms and orchestrate production of cytokines to resolve infection, but these pathways can also recognize self-nucleic acid, resulting in autoimmunity. Nucleic acid sensors can detect DNA in the cellular cytosol. Utilizing an animal model in which there is accrual of cytosolic DNA due to deficiency in the enzyme DNase II, we have studied the impact of excess DNA on inflammatory arthritis and the production of anti-nuclear antibodies.
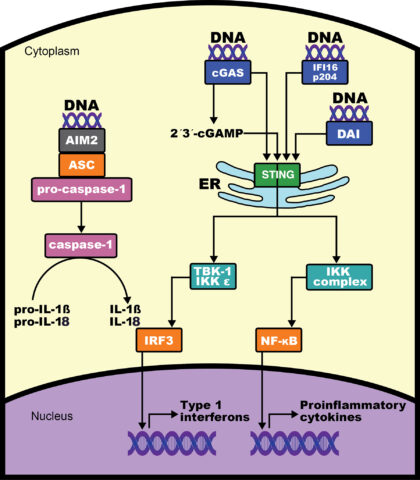
DNA can activate three distinct pathways, each of which contributes differentially to the development of autoimmunity. The Stimulator of INterferon Genes (STING) pathway is essential for the development of arthritis in the DNase II deficient mouse, while the Absent in Melanoma 2 (AIM2) pathway contributes, but is not sufficient for, arthritis development, due to decreased production of IL-18. Deficiency of the endosomal TLR pathway does not affect arthritis severity, but completely abrogates the development of anti-nuclear antibodies in the DNaseII deficient setting. We continue to address the role of these pathways in autoimmune diseases. In addition, several projects in the laboratory, highlighted below, address the specific role of these innate immune pathways in bone.
DNaseII deficient mice exhibit striking trabecular bone formation in the spleens and long bones, and this bone accrual phenotype requires STING, leading us to investigate the role of recognition of DNA in bone. DNA found in the cytosol of cells is a danger signal, indicative of viral infection or response to oxidative stress and clearance of apoptotic cells. Cytosolic DNA can be recognized by several pathways, including the cGAS (cyclic GMP AMP synthase)-STING (STimulator of INterferon Genes) pathway. Activating mutations in STING lead to the type I interferonopathy SAVI (STING-Associated Vasculopathy with onset at Infancy). We are interested in understanding how this pathway influences bone homeostasis, with particular interest in the role of STING in modifying bone loss.
AIM2 is a DNA-sensor that is capable of determining whether a cell lives or dies. Upon activation, AIM2 assembles an inflammasome complex and activates the mature form of IL-1β and IL-18. AIM2 can also induce pyroptosis, an inflammatory form of cell death through the activation of Gasdermin D. We are investigating the role of AIM2 in inflammation and bone homeostasis during aging and cancer.
IL-17A is a key inflammatory cytokine in certain rheumatic diseases, including spondyloarthritis and psoriasis. While studying IL-17A biology in the immunopathology of SpA, we became interested in additional IL-17 family members and are investigating their role in the rheumatic diseases and in bone pathology.
Rheumatoid arthritis patients are at a nearly doubled risk of developing cardiovascular disease (CVD), which is not fully controlled by traditional CVD or RA therapies. There remains an unmet clinical need to pursue the shared pathologies connecting RA and CVD. We are interested in the role of one factor, the epigenetic regulator of hematopoietic fate Ten-Eleven Translocase 2 (TET2) and are studying the potential role of TET2 in arthritis, bone and the vasculature.